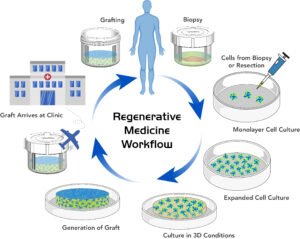
 In the fields of tissue processing and transplantation, tissue engineering, and regenerative medicine, there is a need for containers of appropriate quality which can be used from R&D all the way through product commercialization. Quite often, containers used at the R&D phase are made from laboratory grade materials like polycarbonate, polystyrene, and polypropylene. These containers are not designed for commercial processes, lack a complete package of USP biocompatibility and extractables testing, and can leak during transport.
In the fields of tissue processing and transplantation, tissue engineering, and regenerative medicine, there is a need for containers of appropriate quality which can be used from R&D all the way through product commercialization. Quite often, containers used at the R&D phase are made from laboratory grade materials like polycarbonate, polystyrene, and polypropylene. These containers are not designed for commercial processes, lack a complete package of USP biocompatibility and extractables testing, and can leak during transport.
An increasing number of tissue engineering and regenerative medicine companies are also finding that contaminants leached from the container impact the viability of live tissues and cell cultures. Biopsies taken from patients could be damaged beyond reasonable recovery when stored and transported from the clinic to the cell therapy facility. The more valuable finished transplant tissue can also be damaged by contaminants from the container during transport back to the patient.
Purillex® containers are molded from high purity virgin PFA, which has the lowest extractables and leachables profile of any polymer and has been shown to dramatically improve tissue and cell health compared to commodity containers molded from other polymers. This means you can use the same container from research through commercial and increased speed to market for your therapeutic. Purillex containers have been identified as a critical component for an FDA-approved, commercial autologous tissue transplant, where they are making a positive difference in patient outcomes.
Tested for Leak-Free Performance
Savillex has performed comprehensive testing on all Purillex products, including pressure decay, ASTM leakproof and shipping tests, vacuum resistance, and pressure burst tests. Test data is available upon request. In addition, Purillex products are Class VI, have undergone complete USP testing, and come with manufacturing lot certification and full support for bioprocess and cell therapy applications.
Key Benefits of Purillex® Containers for Tissue Handling and Transport
- Extremely low extractables and leachables levels
- Non-stick and non-reactive surface – gentle to live tissues and cells
- High integrity seal across a wide temperature range, with supporting CCIT data
- Inert to virtually every solvent and chemical
- Available clean packaged, autoclaved to a SAL of 10-6 and ready-to-use
- Long shelf life
- Validation package and full manufacturing lot traceability
- Passed ASTM D4991-07(2015) standard test method for leakage testing of empty rigid containers by vacuum method
Download our product Data Sheet to learn more about the complete line up of Purillex containers designed for tissue handling and transport.