 Understanding the impact of container extractables
Understanding the impact of container extractables
When choosing containers for applications such as critical drug substances, cell therapies and regenerative medicines, it’s important to consider the effect extractables can have on products.
Extractables are the organic or inorganic chemical compounds released from container systems when faced with aggressive laboratory conditions (i.e., elevated temperatures, harsh solvents). Under standard storage and use conditions, these extractables could potentially migrate into drug products over time as leachables. So, if extractable compounds exist in container systems, they need to be carefully examined for potential characterization as leachables based on their concentration.
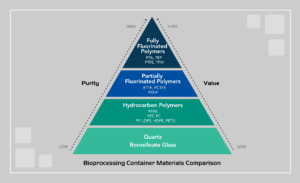
Fluoropolymers the materials of choice for high purity applications
Fluoropolymers may be the highest purity materials currently available for drug substance storage applications. In fact, fluoropolymer vials made from PPE and PFA are used to store QC control samples during extractables screening protocols. What’s more – the use of fluoropolymer vials is specifically called out in several industry-standard procedures.
Extractables profile study for fluoropolymer bottles
Recently, a major biopharmaceutical company conducted a study aimed at establishing an extractable profile for fluoropolymer bottles. The study used aggressive extraction conditions that were specifically chosen to bracket drug product applications.
To view the full extractables study process and results, be sure to check out our technical note.
And while you’re here, there’s much more we would like to show you about our lineup of ultra-pure fluoropolymer vials for bioprocess. Get full product details and technical specifications now.



