Shipping failures happen…and the consequences can be costly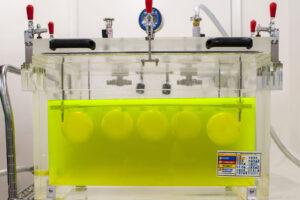
Many of our customers in the tissue engineering and cell therapy fields come to us when their standard, off-the-shelf labware fails during shipping.
Most of these failures appear in the form of liquid leaks after air shipments. This is a significant issue, as live tissue or cell samples that get damaged in transit can lead to surgical delays and poor patient outcomes.
That’s why, when selecting containers for critical CGT (Cell and Gene Therapy) applications, it’s crucial that containers are tested to ASTM shipping test standards.
Setting the standard for shipping
The ASTM D4991-07 (2015) Standard Test Method for Leakage Testing of Empty Rigid Containers by Vacuum Method is a standard testing protocol designed for empty rigid containers under differential pressure conditions – much like those that can occur during air transport.
ASTM D4991-07 is an essential test for companies considering the use of bottles, jars, vials – or other containers – for critical shipping applications that require sterility to be maintained or involve hazardous liquids.
So, it shouldn’t come as much of a surprise that ASTM is an extremely difficult test for most containers – let alone standard, off-the-shelf containers, to pass.
Naturally, we wanted to get a better idea of how our own products would fare and tested Savillex Purillex® bottles and jars at an independent lab to see if they met the rigorous ASTM D4991 requirements. Read our technical note for the full set of test results.



